Background
Early cardiotoxicity de-risking of small molecules such as kinase inhibitors can expedite the selection of clinical candidate compounds. Currently ICHS7A/S7B guidance primarily addresses repolarization delay while contractility reduction is rarely assessed even in IND-enabling studies.
The CiPA initiative is a multi-discipline in vitro approach to assess the potential for arrhythmias (1), including the use of human induced pluripotent stem cell-derived cardiomyocytes (hiPSCCM).
In 2020, the Safety Pharmacology Society (SPS) published a survey that revealed that only 23% of SPS responders used hiPSC-CM to examine drug-induced toxicity (2).
Use of the hiPSC-CM assay has several advantages such as (a) an intact electrical conduction, (b) contractile components, and (c) human cardiac cell origin which provides a derisking approach and human translatability compared to patch clamp assays utilizing transfected cell lines with single ion channels.
Case Study 1
The hiPSC-CM (cardiomyocyte) assay was able to detect electrical conduction signal changes beyond hERG inhibition.
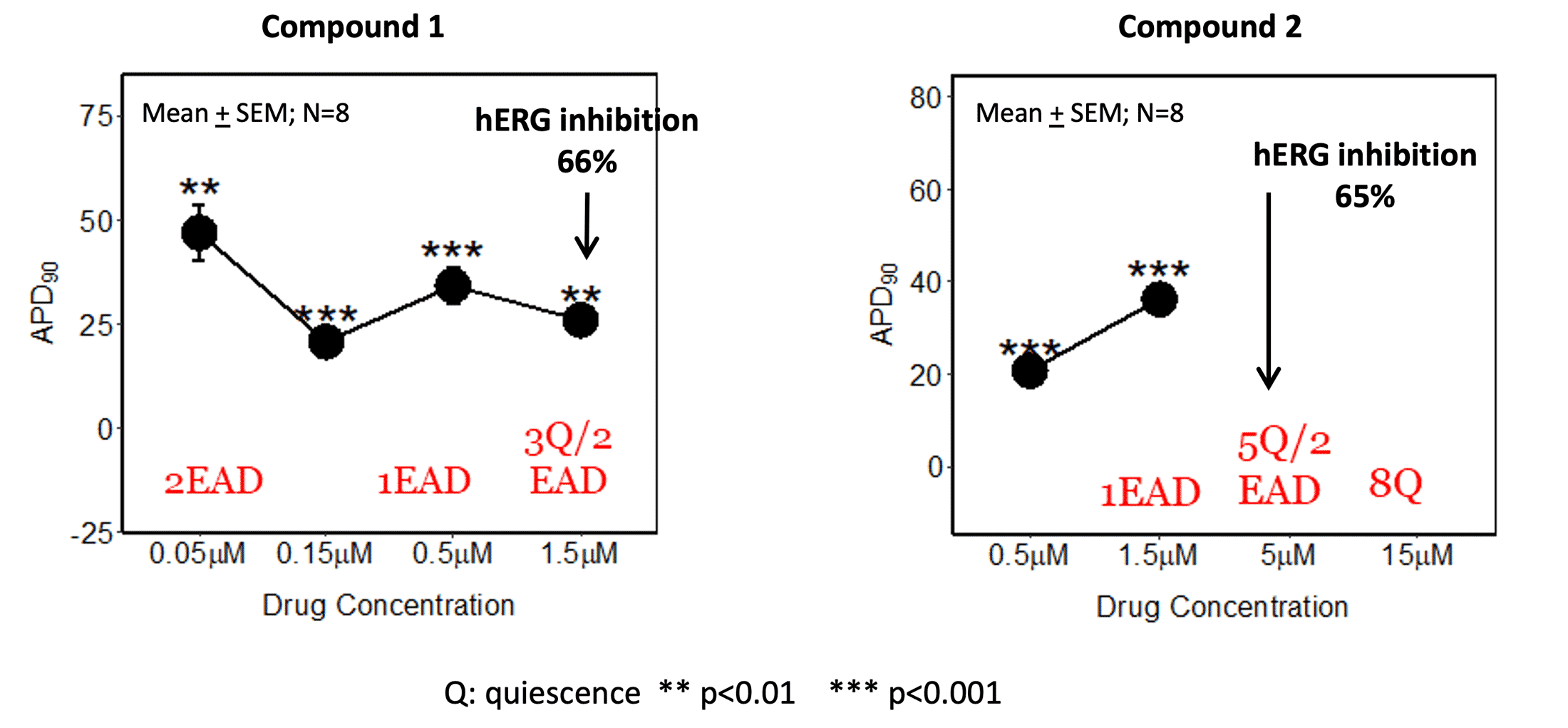
HiPSC-CM (Figures 1 and 2)
Early after depolarization (EAD) was detected in HiPSC-CM (performed at Clyde Biosciences) compared to the hERG IC50 (patch clamp at 37°C).
Figure 1. HiPSC-CM action potential (AP) duration and EAD compared to hERG IC50 (both AP measurements and patch clamp assay at 37°C)
AP measurements performed by Clyde Biosciences
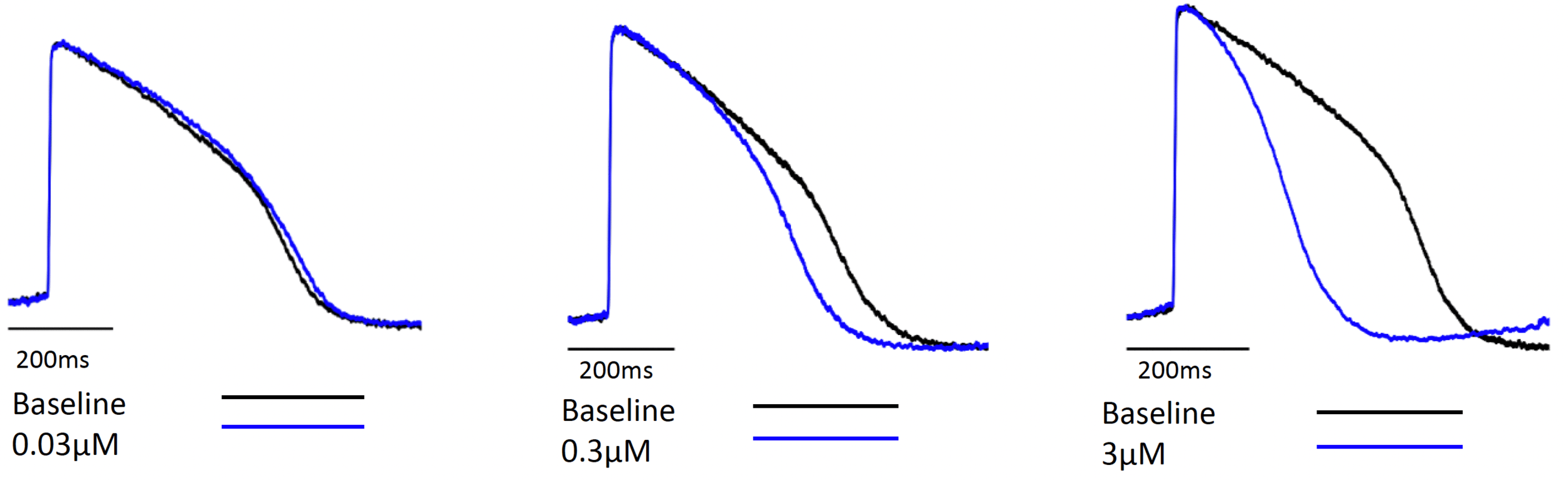
Figure 2. Representative tracings
Table 1. Screening Patch clamp/fluorescence IC50 inhibition
(performed at Pharmaron)
Case Study 1 Conclusion
HiPSC-CM may be more sensitive in detecting conduction liabilities as compound 2 had EADs and quiescence at concentrations below the hERG IC50. Additional ion channel activity (Table 1) in addition to hERG inhibition might contribute to the observed effects in hiPSC-CM. As these additional ion channels are less commonly tested, the cardiomyocyte assay can serve as a reliable tool for integrated ion channel activity.
Case Study 2
Comparison of hiPSC-CM APD and other electrophysiology parameters for prediction of conduction and contractility changes in ex vivo and in vivo studies.
HiPSC-CM (Figures 3 and 4)
Compound 3 elicited significant decreases of action potential duration (APD) in the cardiomyocyte assay. This compound also inhibited Cav1.2 inhibition activity (IC50 = 0.5 μM).
Figure 3. hiPSC-CM APD change
Figure 4. Compound 3 concentration dependent hiPSC-CM APD shortening representative tracings (provided by Clyde Biosciences Ltd).
Langendorff assay (Figures 5-8)
Compound 3 reduced left ventricular systolic pressure and contractility in isolated female Guinea pig hearts similar to that seen with the L-type calcium channel blocker, diltiazem, in male Guinea pig isolated hearts (performed at CorDynamics).
Figure 5. Left Ventricular Systolic pressure
Figure 6. Contractility (dP/dtmax)
Figure 7. Diltiazem Left Ventricular Systolic pressure
Figure 8. Diltiazem Contractility (dP/dtmax)
Telemetry (Figures 9 and 10)
Compound 3 dose dependently reduced systolic blood pressure with no changes in QTc in telemetry-implanted dogs (LSMean; n = 4) (Study performed at Charles River lab). Dose-dependent decreases in Mean and Diastolic pressure along with compensatory increases in Heart Rate were observed (data not shown). No relevant electrocardiographic interval changes or arrhythmias were noted (QTc results shown).
Figure 9. Systolic Blood Pressure
Figure 10. Heart rate corrected QT (QTc)
Heart rate corrected QT (QTc)
Case Study 2 Conclusion
HiPSC-CM APD shortening translated across hierarchy into blood pressure changes and contractility signals in ex vivo isolated heart assay as well as into the in vivo telemetry study.
Compound Screening in hiPSC-CM assay (2 concentrations) during Lead Identification
Selected measurements summarized below for representative Gossamer compounds. Reference compounds (only 3 shown) at similar screening concentrations. Two concentrations were sufficient to identify potential cardiac liabilities. A full concentration curve can be generated to confirm IC50s. Assay can identify calcium and sodium channel blockers in addition to depolarization delay and TdP risk.
Conc: Concentration; EAD: Early after depolarization; Tachy: tachycardia; Triang: triangulation; TdP: Torsades de Pointes
Summary
Gossamer’s case studies demonstrate that;
1
Cardiomyocyte (hiPSC-CM) assay employed by Clyde Biosciences Ltd using the CellOPTIQ platform can serve as a reliable functional tool for integrated ion channel activity, which are not detected by single ion channel assays.
2
Cardiomyocyte assay can be used to predict electrical physiology changes, as well negative ionotropic contractility changes which correlated well with ex vivo and in vivo study results.
3
Two concentrations in cardiomyocyte assay is sufficient to identify potential cardiac liabilities.
4
HiPSC-CM are translatable to patients while reducing the use of surrogate animal models, thus reducing the use of animals.
References
(1) Pfeiffer-Kaushik ER, Smith GL, Cai B, et al. Electrophysiological characterization of drug response in hSC-derived cardiomyocytes using voltage-sensitive optical platforms. J Pharmacol Toxicol Methods. 2019;99:106612.
(2) Authier S, Abernathy MM, Correll K, et al. An Industry Survey With Focus on Cardiovascular Safety Pharmacology Study Design and Data Interpretation. Int J Toxicol. 2020;39(4):274-293.
Acknowledgements: We would like to thank Dr. Godfrey Smith from Clyde Biosciences and Dr. Liomar Neves from CorDynamics for their scientific expertise in protocol development, data interpretation and discussions.
This poster was authored by Beibei Cai and Kristy D. Bruse of Gossamer Bio, Inc.